- Medtech Chopsticks
- Posts
- 🍜 China’s 2025 Update on Clinical Evaluation Exemption
🍜 China’s 2025 Update on Clinical Evaluation Exemption
Is your device on the list?

The NMPA just released an updated list of medical devices that are exempt from clinical evaluation.
In 2023, the list included 1,025 devices. This year, 22 additional devices were added.
The NMPA updates this exemption list every two years.
It would be ideal if one set of technical documents could satisfy regulators worldwide.
Unfortunately, that’s still not the case.
Some regulators accept clinical evaluation reports from the EU or U.S., but China isn’t one of them.
It’s not the end of the world.
Let’s find out whether your device is now exempt from clinical evaluation.
🍜 Devices Exempt from Clinical Evaluation
Here’s a look at the 22 new devices that made it onto the exemption list.
The devices are sorted by product category and classification code, and all of them are Class II or Class III.
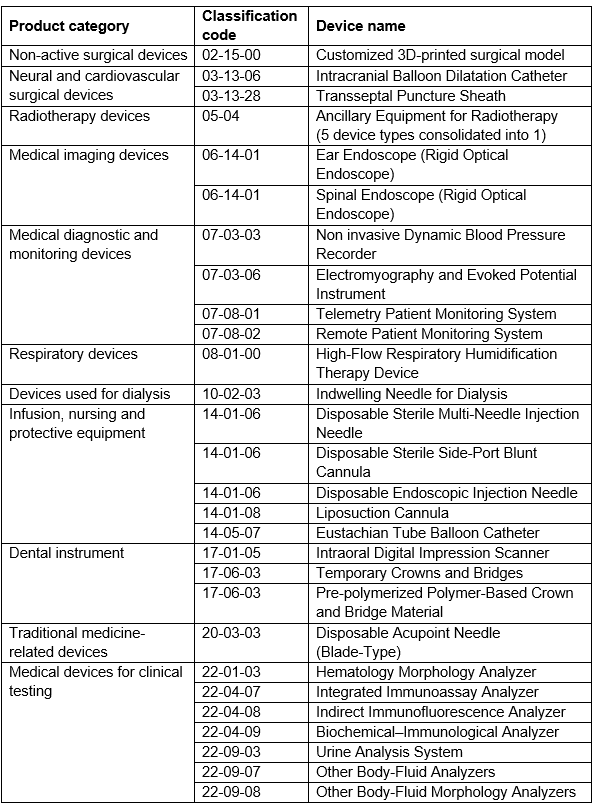
Click here to download the full list directly from NMPA.
What qualifies a device for exemption?
The NMPA grants exemptions to devices with mature designs and well-defined risk profiles.
In other words:
The mechanism of action must be clear and well understood.
The manufacturing process should be well established.
Product designs need to be consistent and robust.
Similar devices should have been on the market for years without any serious adverse events.
Intended use cannot deviate from the scope stipulated by the NMPA.
If your device doesn’t fit this picture, you’ll need strong non-clinical data to prove the safety and performance.
🍜 What Does This Mean For You?
Is your device now exempt from clinical evaluation in China? Let’s check. Sometimes a second opinion can save you months of work.
If you’re still in the planning stage or preparing your documents, this is good news. Below is a simple flowchart showing the clinical evaluation options:

If your product qualifies for an exemption, you save significant time and resources.
In that case, all you need to submit is a Comparison Analysis with suitable predicate device(s).
Click here to download the template. It’s free!
However, if you've already submitted your registration dossier, the exemption won’t apply retroactively.
In fact, during the NMPA feedback process, they may now ask you to supply a comparison table instead of a clinical evaluation.
I’ve laid out the possible clinical routes of medical devices in my previous post. Check out the link at the end of this newsletter if you’re interested.
Need advice or step-by-step support on your clinical evaluation strategy? Let’s chat. Or feel free to reach out via email or LinkedIn.
PS: I currently have capacity to take on a new consultation project. Schedule a call here.
🍜 Bonus Reading
Official NMPA notice on the Release of the Catalogue of Medical Devices Exempted from Clinical Evaluation (Chinese only)
Previous post on the Clinical Evaluation Routes for Medical Devices in China
Disclaimer: This content is created solely in my personal interest and does not reflect the views, opinions, or policies of my employer or any other organizations I am affiliated with. The information provided is for informational purposes. Neither I nor my employer is liable for any use of this content. This newsletter does not compete with my employer’s interests. Any references to regulatory updates or guidance are based on publicly available information and are not intended to infringe upon proprietary or confidential content.