- Medtech Chopsticks
- Posts
- 🍜 China's UDI Drafts in 2025
🍜 China's UDI Drafts in 2025
New Deadlines, Exemptions, and a Surprise Link to NHSA

China’s NMPA just released two new drafts on how Unique Device Identification (UDI) will be rolled out nationwide:
UDI Implementation of Medical Devices in Specific Situations
UDI Implementation of Medical Devices for Subsequent Products
Let’s take a look at the new deadlines, requirements, and what it means for you as a manufacturer.
🍜 UDI Deadlines
China’s first UDI regulation was published in 2019.
That rule defines who’s responsible for what, from manufacturers to the coding agencies.
But here’s the catch: there were no timelines uh?
Since then, NMPA has run three pilot phases. It mainly covered high-risk Class III and selected Class II devices.
Now, the 2025 draft rules go further. They aim to be mandatory for all device classes with the following key timelines:
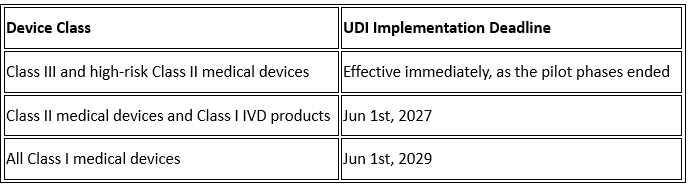
Here is what you should know:
Starting from the new effective dates, manufacturers must submit UDI details of the smallest sales unit when registering medical devices.
For already approved products, the UDI information should be added at the time of registration amendment or renewal.
UDI is optional for the devices manufactured before the implementation date.
The cut-off is based on the manufacturing date, not the import date.
A change in product identification (like UDI) is not a registration item category, so a registration update is not needed just for that.
Manufacturers must upload UDI data of the smallest sales unit or higher packaging level to the UDI database before the product hits the Chinese market.
In the next sections, we’ll explore further requirements derived from the new drafts.
🍜 UDI Exempted Devices
Not every product needs a UDI label. NMPA’s new drafts outline when UDI can be exempted.
🔺Smallest Sales Package with Multiple Products
When a smallest sales unit contains several individual items, each with its own model or batch number, you don’t need to label each one separately.
One UDI for the entire sales package is enough.
Great examples are medical masks or condoms. Each piece doesn’t require its own UDI.
🔺Disposable Devices Inside a Medical Device Kit
If a single-use device is meant to be used only with the kit, it can skip UDI labeling.
And if that single-use device is already on the exempt list, it stays exempt even when it’s a part of a kit.
However, the kit itself must have its own UDI.
🔺Drug-device Combination Products
Combination products where the drug provides the primary mode of action can be exempt from UDI, if the drug component is already traceable.
🔺Custom-made Medical Devices
Custom-made devices, as defined in the Regulations on the Supervision and Administration of Custom-Made Medical Devices, are fully exempt from UDI.
🍜 Reusable Medical Devices
China’s new drafts also set special rules for reusable devices.
UDI-DI is mandatory for Class I reusable surgical instruments. But the UDI-PI remains optional.
Otherwise, the overall requirements are aligned with Part C of Annex VI under the EU MDR.
That means the UDI must remain readable after each reprocessing cycle and last throughout the device’s intended lifetime.
If direct marking could affect the device’s safety or performance or if it’s not technically feasible, you can place the UDI on the packaging or label instead.
For manufacturers who have already implemented UDI, there is flexibility.
If your device falls under a UDI exemption in China, you can choose whether to keep or remove the UDI marking.
In all cases, the registrant or authorized representative must upload the relevant UDI data to China’s Medical Device UDI Database.
🍜 Bonus Reading: EU vs. China
As EU manufacturers, you may wonder how China’s UDI system differs from the one we know in the EU.
🔻Concept
In the EU, the MDR introduced three aspects: Basic UDI-DI, UDI-DI and UDI-PI.
Although basic UDI-DI never appears on the label or packaging, it breathes “quietly” in your Declaration of Conformity (DoC) and technical documentation.
However, there is no such concept of basic UDI-DI in China. Each UDI directly identifies the product level that’s placed on the market.
🔻UDI Issuing Entities
According to the Implementing Decision (EU) 2019/939 and (EU) 2024/2120, there are four issuing entities (GS1, HIBCC, ICCBBA, IFA) authorized until 27 June 2029.
In China, NMPA has only accredited three institutions so far:
Among these, GS1 is the only one with international recognition.
It’s smarter to choose a universally recognized code issuer if you’re selling globally.
🔻China’s NHSA Consumables Codes
NHSA (National Healthcare Security Administration) assigns a national code to every medical consumable.
This code acts as a universal language across China’s healthcare system.
It links insurance, procurement, hospital management, and national data platforms under one identifier.
2025 UDI implementation drafts officially connect NHSA codes with UDI.
This means that medical device operators (e.g. distributors, hospitals, etc.) must ensure that the data in both systems match and remain traceable.
For manufacturers, this adds another step.
You’ll also need to include the NHSA classification and consumables code (if applicable) when entering data into the NMPA UDI database.
It’s a move that ties regulatory compliance to medical reimbursement and procurement.
🔻Public UDI Database & Access
In the EU, EUDAMED is designed to connect national competent authorities and the European Commission, while also providing public access to certain data.
Manufacturers must register their devices and UDI information within the specified deadlines.
But, not every EUDAMED module is fully live yet.
China’s national UDI database also allows public access, where anyone can look up product information by UDI.
This database doesn’t specify all economic operators, which is required in EUDAMED.
You can explore it here: https://udi.nmpa.gov.cn/download.html
Find this insightful? Don’t forget to subscribe.