- Medtech Chopsticks
- Posts
- 🍜Journey of Innovative Medical Devices
🍜Journey of Innovative Medical Devices
What makes China’s pathway different from the US?

Over the last five years, the FDA has issued around 140 - 210 Breakthrough Device designations each year.
One standout recipient is Argentum Medical, LLC (acquired by Bravida Medical, LLC), a pioneer in silver-based antimicrobial technology.
Its Silverlon® dressing secured Breakthrough Device designations for treating radiation dermatitis (RD) and cutaneous radiation injury (CRI) with dry and moist desquamation. Link to the FDA at the end.
This was the first U.S. clearance for a dressing specifically targeting CRI.
RD and CRI can progress to fibrosis and ulceration, leaving lifelong scars. Topical creams and standard dressings offer some relief.
But, none had been cleared to stay on a radiologically injured wound for up to seven days.
Silverlon fills that gap by allowing for fewer and less painful dressing changes.
The result? A better outcome and a higher quality of life for patients.
Today we’ll compare the innovative pathways between the US and China.
🍜 Innovative Pathways in the US
A product must meet two key criteria under the FDA’s Breakthrough Devices Program:
Clinical Impact
The device provides a more effective treatment or diagnosis of life-threatening or irreversibly debilitating human diseases or conditions.
Technological Edge
The device should either: introduce a breakthrough technology, deliver a significant advantage over cleared alternatives, address a condition with no cleared alternatives, or serve the best interests of patients.
The manufacturer goes for the De Novo pathway for novel, low- to moderate-risk devices without a predicate.
Breakthrough accelerates review for high-impact technologies, while De Novo creates a new classification for first-of-kind, lower-risk products.
How does this compare with China’s Innovative Device pathway? Let’s explore next.
🍜 Innovative Pathway in China
China’s NMPA first rolled out its Special Review and Approval Procedure for Innovative Medical Devices in 2018.
Demand keeps rising.
In 2024, the NMPA received 451 innovative-device applications. 65 devices received approvals. The acceptance rate is between 15-20 %.
Does your product have the novelty and clinical value to make the cut? Let’s find out!
✅ Do you hold a core technology patent in China?
You must own an invention patent for the product or be authorized to use the patent via a transfer agreement.
A published patent application can also work if the State Council’s patent office has issued a novelty report confirming the technology’s innovation.
Main difference: patent ownership isn’t a prerequisite to quality for the innovative pathways in the US.
Timing matters! You must file for the innovative pathway within five years of the patent’s grant date.
✅Is the working principle first-of-its-kind?
Being innovative alone is not enough. The device must introduce a mechanism or technology not yet approved domestically.
The safety and performance must be significantly better than similar devices.
The adopted technology should align with international standards and offer meaningful clinical value.
Perform a risk-benefit analysis to evaluate whether the benefits outweigh the potential risks.
✅ Have you done a preliminary study and built a functional prototype?
NMPA expects to see that the manufacturer has done considerable research on the potential risks, safety and performance indicators.
The applicant must establish a controlled research procedure to ensure the authenticity of the data sources.
Research data must be complete, authentic and traceable.
🍜 What Do You Need?
In addition to the standard Class II/III registration dossier, the NMPA expects an “innovation packet” that proves your device qualifies for the Special Review pathway.
It includes:
IP-supporting Evidence
Patents, license or transfer agreements, and any supporting certificates.
Development Overview
Summary of the development process, product designs, and clinical or laboratory research.
Product Innovation
Evidence and officially published peer-reviewed papers, monographs, and literature reviews that show clinical application.
Provide a comparative analysis of similar products on the Chinese or international markets, if any.
NMPA will also review other documents that fall within the submission dossiers of Class II and III Initial registration. Such as:
Product technical requirements (PTR)
Risk management file
Instruction for Use (draft)
Declaration of document authenticity
Overseas manufacturers must apply through their China subsidiary or an authorized representative.
Choose wisely if you opt for an in-country authorized representative who is independent of your company.
🍜 Screening Timeline
The process usually takes around 90 -110 working days, including administrative timelines.
The detailed breakdown is as below:
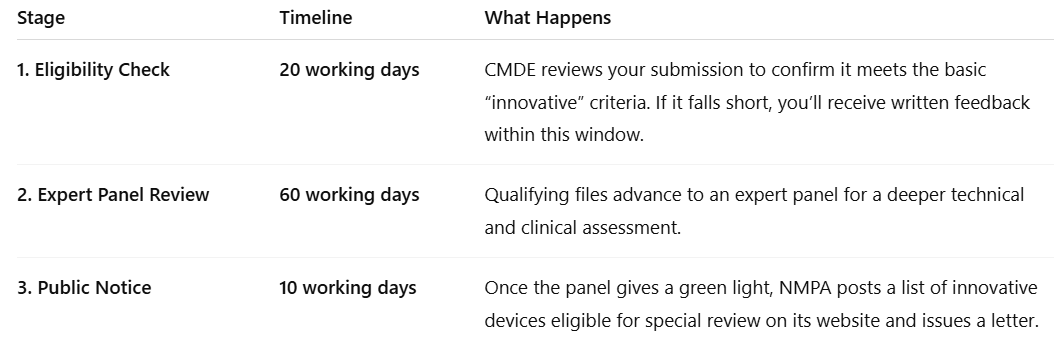
You can file an appeal for re-evaluation if your application is rejected.
🍜 Benefits of Special Review Pathway
Getting into NMPA’s Special Review channel comes with some perks! You are prioritized in many aspects.
First, your device will be prioritized for type testing at the designated test labs. You will have a shorter waiting time.
The same fast-track treatment also applies when your dossier reaches the technical review stage.
This also applies to the technical review of the registration procedures. The official review timeline will be shorter.
NMPA will assign a dedicated technical reviewer for your application.
You can request a consultation and ask for guidance before the submission or during the technical review stages.
The reviewer will pull in additional subject-matter experts as needed.
Most companies use these sessions to address and discuss clinical trial designs, performance benchmarks, and any potential safety issues.
Let’s chat if you’re not sure if the Innovative Device pathway is the right fit for you.
🍜 Bonus Reading
Official notice on the "Implementation Rules for the Special Review Application for Innovative Medical Devices” (Chinese only)
US FDA 510(k) summary of Silverlon® Wound Contact, Burn Contact Dressing
Disclaimer: This content is created solely in my personal interest and does not reflect the views, opinions, or policies of my employer or any other organizations I am affiliated with. The information provided is for informational purposes and should not be construed as professional advice. Neither I nor my employer is liable for any use of this content. This newsletter does not compete with my employer’s interests. Any references to regulatory updates or guidance are based on publicly available information and are not intended to infringe upon proprietary or confidential content.